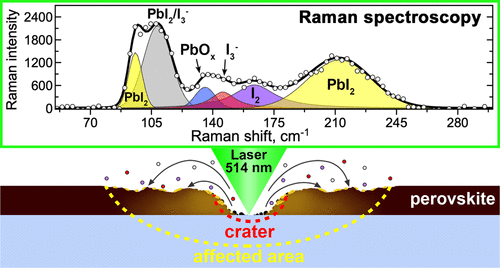
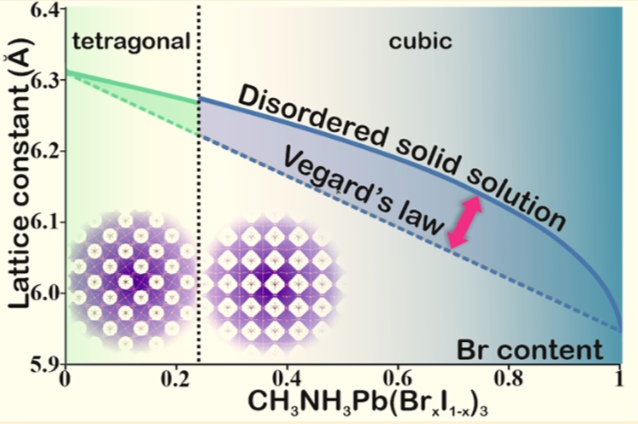
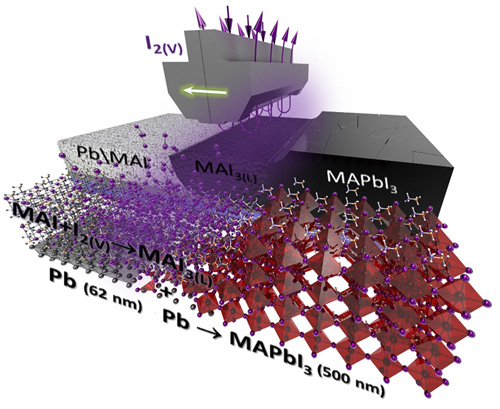
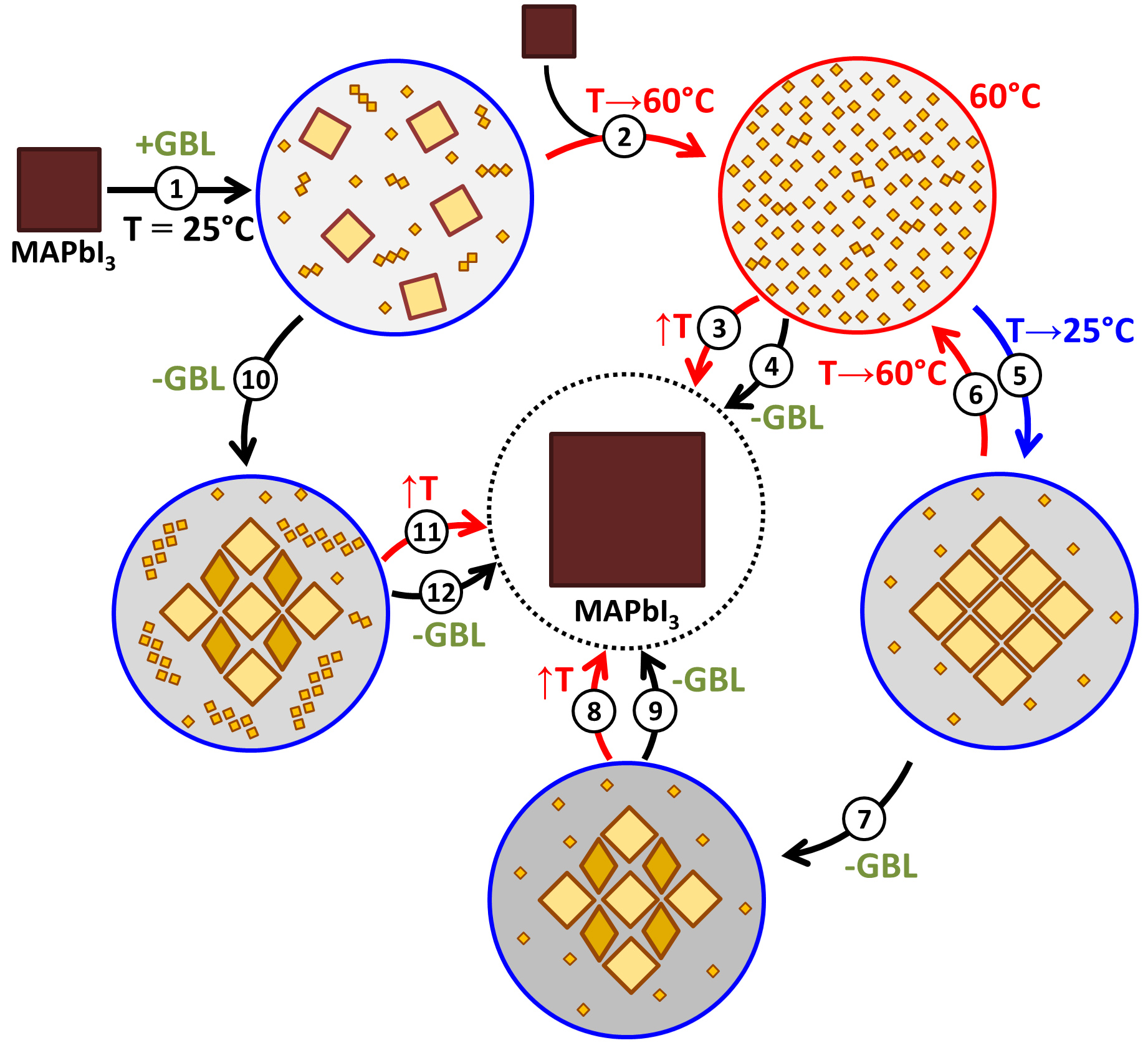
In 2019, the 150th anniversary of the discovery of Periodic law of chemical elements by D.I.Mendeleev, and the year is offilically declared by UNESCO as the International Year of the Periodic Table of Chemical Elements. Throughout the year, activities dedicated to this event will take place all over the world.
On January 29, at the UNESCO headquarters in Paris, and a week later, on February 6, at the Russian Academy of Sciences in Moscow the opening ceremony of the year was held with the participation of the Russian Minister of Science and Higher Education M.M. Kotyukov, President of the Russian Academy of Sciences Academician A.M. Sergeev, Rector of Moscow State University Academician V.A. Sadovnichy and the Chairman of the Government of the Russian Federation D.A. Medvedev. Students and employees of the Laboratory of New Materials for Solar Energetics organized an interactive experimental exhibition “Laboratory” dedicated to the application of various chemical elements in chemistry and materials science.
The laboratory of new materials for solar energetics presented at the exhibition three chemical elements that became key in the development of solar cells of new generation: ruthenium (Ru) - an element discovered in 1844 at Kazan University and named after Russia, and also iodine (I) and lead (Pb) - the elements of hybrid perovskite, the namesake of a mineral discovered five years earlier in the Ural Mountains and named after Count Lev Perovsky.
Organic ruthenium-based complexes effectively absorb sunlight and are used in dye sensitized solar cells (DSSC, or the so-called “Gretzel cells”), while hybrid perovskites based on lead iodide is the main component of perovskite solar cells.
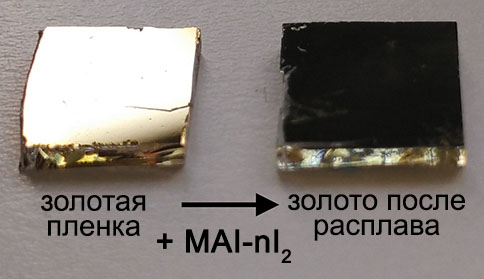
All visitors to the exhibition could create their own solar cell and learn about the principles of DSSC and perovskite solar cells. Head of the laboratory A.B. Tarasov demonstrated to the delegation of the leadership of the Russian Academy of Sciences and the Government of the Russian Federation the success of Moscow State University in the field of modern solar energetics: Alexander Sergeev assembled a solar cell based on a ruthenium complex, and Dmitry Medvedev obtained a perovskite film by converting a layer of metallic lead by a reactionary melt of polyiodides using the technology developed in the laboratory, highly appreciating the level of Russian research in this field.
In addition, the exhibition featured stands devoted to materials for chemical current sources (hydrogen and lithium chemical elements), high-temperature superconducting materials (nitrogen), modern compounds for agriculture (potassium and phosphorus), luminescent materials (lanthanides), nanostructured materials based on aluminum (aluminum).
The NAUKA 0+ science festival, which is organized annually by Moscow State University, this year will be devoted to the Periodic Table of Chemical Elements of D.I. Mendeleev.