The Bachelor's program in “Chemistry, Physics and Mechanics of Materials” has been implemented in 2017 by Moscow State University at the Shenzhen MSU-BIT University under the guidance of the Dean of the Faculty of Materials Science, academician K.A. Solntsev. This year, the first students of the program completed their second-year bachelor studies with a successful defense of the coursework in inorganic chemistry, which was held on July 13, 2019.
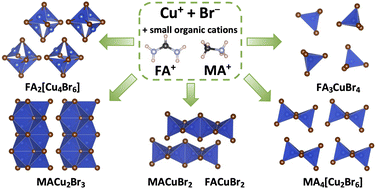
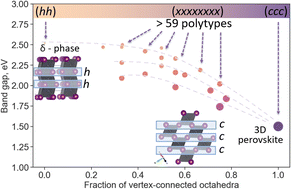
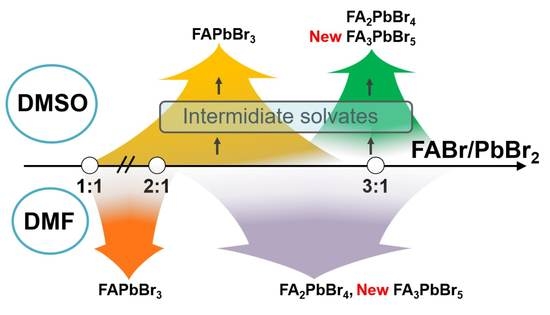
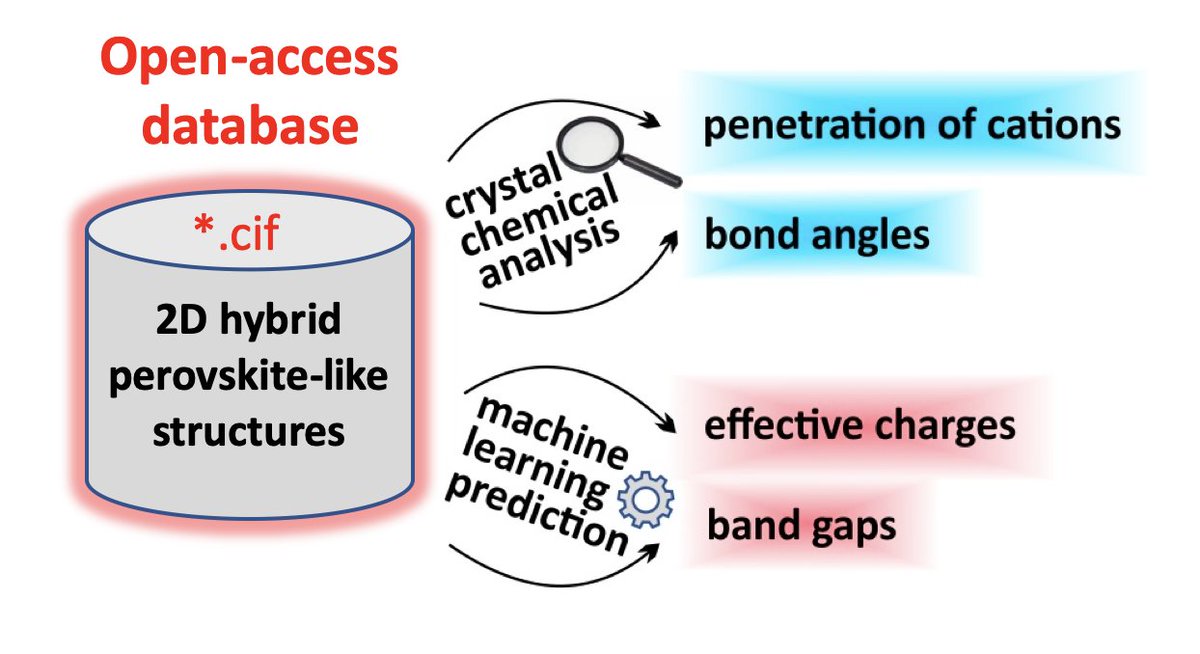
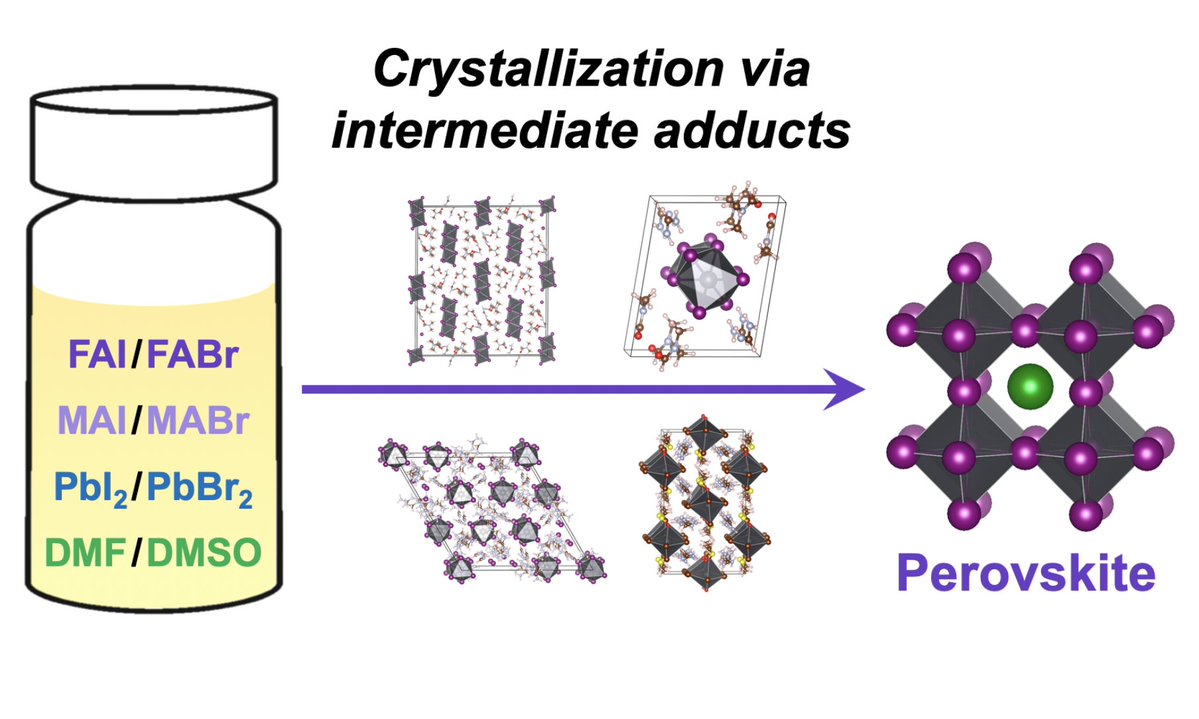
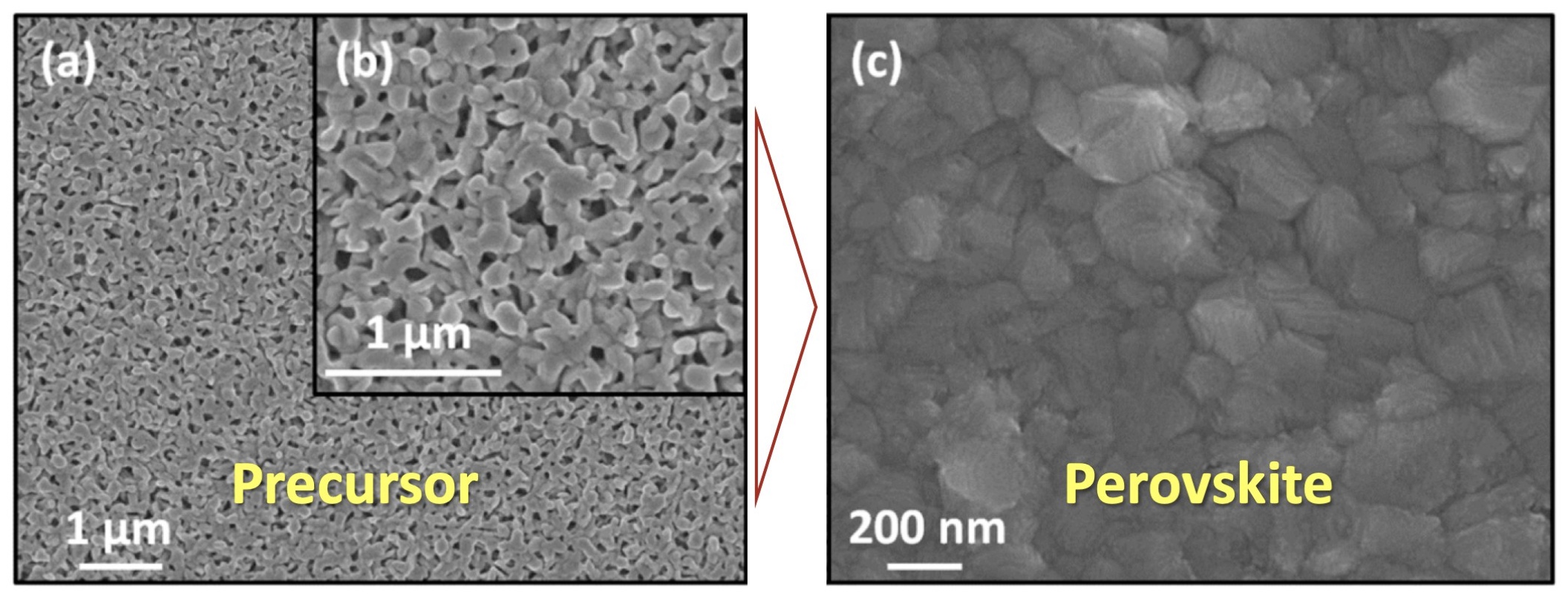
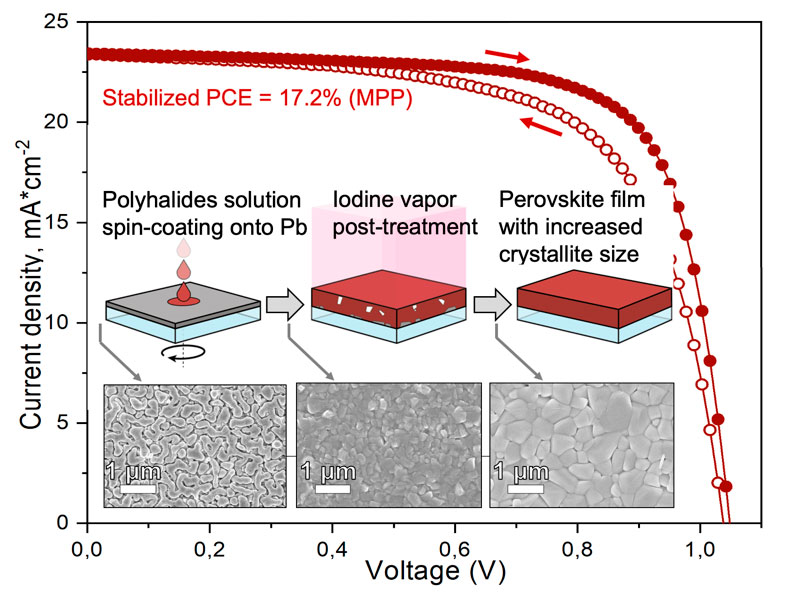
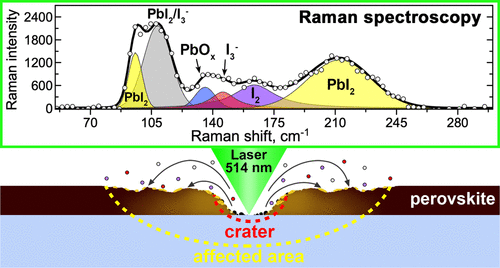
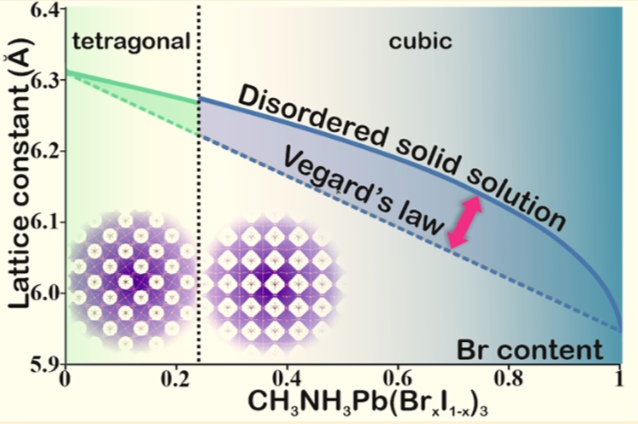
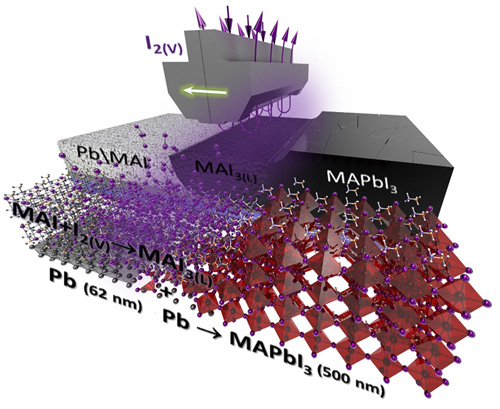
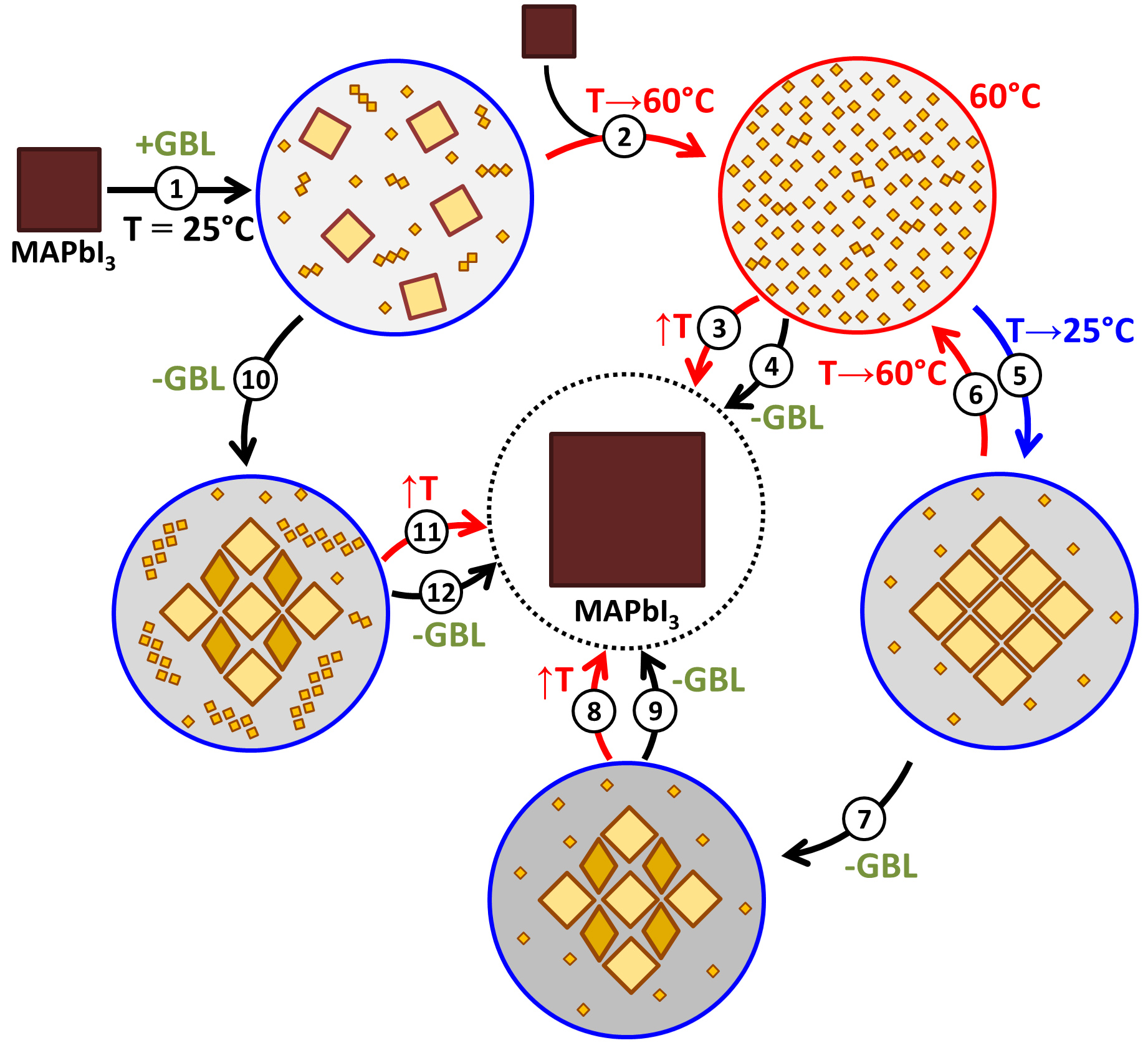
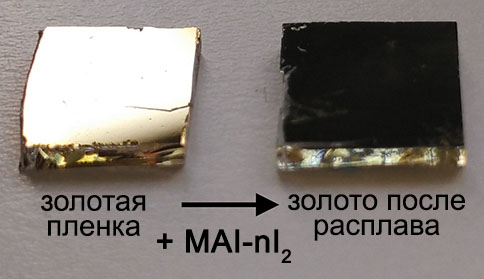
While doing the coursework, the students have had the first experience of performing scientific research and solving serious scientific problems. The discussion with the supervisors was held completely in Russian, which the students had managed to learn during three previous semesters of studying at the Shenzhen MSU-BIT University. Carrying out the coursework, the students learned about the current trends in perovskite photovoltaics and studied the basics of various characterization methods, such as X-ray diffraction, diffuse reflection spectroscopy, differential scanning calorimetry and scanning electron microscopy. The students have successfully applied the gained knowledge to carry out the assigned tasks: the synthesis of perovskite single crystals, the replacement of ions in hybrid perovskites via the ion-exchange method, the study of the crystallization of various perovskite compositions from aprotic solvents and the study of their environmental stability.
Based on the results of the thesis defense, the faculty commission has rated the work of four students as “excellent”; one student received a “good” mark. Course supervisors on perovskite photovoltaics, junior researcher Andrey Petrov and head of the laboratory Dr. Alexey Tarasov pointed out the extraordinary commitment and diligence of the students in carrying out scientific research and wished them further success in science.